In 2016, most cancers biologist Johanna Joyce of the Memorial Sloan Kettering Most cancers Middle printed a research in Science that examined the consequences of a macrophage-targeted remedy on glioblastoma multiforme (GBM), a typical and aggressive mind tumor.1 She discovered that in preclinical mouse fashions, GBM tumors regressed in response to the remedy, however with combined long-term outcomes. About half the time, the tumors would recur. Within the different half, they remained in a completely dormant state.
“In each case the place we discovered a recurrence of the tumor, this was at all times adjoining to a scar that had fashioned within the mind.” Joyce stated. “This actually made us assume, is that this causal or consequential?”
In a latest research printed in Most cancers Cell, Joyce, now on the College of Lausanne, revealed the findings from her group’s investigation into this query.2 The researchers found that fibrotic scar tissue fashioned after macrophage-targeted remedy acts as a secure harbor for dormant most cancers cells and actively contributes to recurrence of resistant GBM. Extra importantly, they discovered that the method of scar formation—and due to this fact, tumor recurrence—could be stopped.

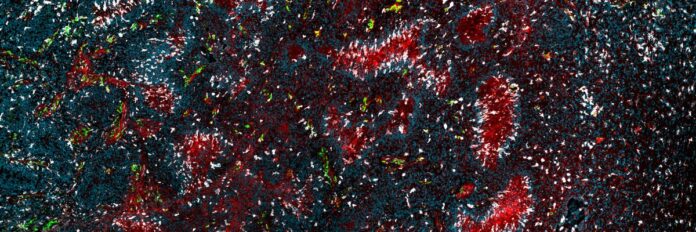
A brand new research revealed that treatment-associated fibrotic scarring contributes on to recurrence of glioblastoma (vasculature in pink) by shielding dormant tumor cells from the immune system (tumor-associated macrophages and microglia proven right here in inexperienced and white).
Professor Johanna Joyce laboratory, College of Lausanne
“This analysis is essential as a result of glioblastoma stays probably the most tough cancers to deal with, with a excessive price of recurrence regardless of intensive therapies. The research sheds gentle on the fibrosis-driven mechanisms that allow tumor cells to outlive publish remedy,” Misty Jenkins, an immunologist on the Walter and Eliza Corridor Institute of Medical Analysis who research mind most cancers and was not concerned within the analysis, wrote in an e mail.
To map out the mobile and molecular terrain of the scar tissue, Joyce and her group utilized an built-in multiomics method to the scar tissue: mass spectrometry-based proteomics, single-cell RNA sequencing, and spatial transcriptomics.
Given the fibrotic nature of the scars, they reasoned that extracellular matrix (ECM) proteins could be outstanding. “We certainly discovered that many various extracellular matrix proteins—many collagens, fibronectin…and so forth—had been enriched within the scar,” Joyce remarked.
Joyce and her group developed a machine studying mannequin that allowed them to combine the outcomes of sequential immunofluorescence experiments performed throughout a variety of time factors publish remedy with the omics knowledge. “By doing this in a time course, in a dynamic method, that actually allowed us to determine the important thing networks throughout the tumor microenvironment, the cell varieties that gave the impression to be the nodes of these networks, how that modified in response to the therapeutic intervention, and the way within the context of recurrence, these networks had been utterly restructured,” Joyce elaborated.

Picture exhibits rendition of a glioblastoma for spatial evaluation, with cells of the tumor microenvironment outlined by computational software program pipeline. Areas of tumor necrosis, immune cell infiltration, and plentiful vascularization could be seen on this picture.
Spencer Watson and Johanna Joyce, College of Lausanne
The outcomes revealed that fibroblast-like cells predominantly drove fibrosis and scar formation in post-treatment tissue, progressively fanning out over the area the place the tumor was and secreting the ECM proteins. The ensuing scar acts as a protumor survival area of interest, harboring any remaining most cancers cells and shielding them from the immune system, however retaining them briefly in a non-proliferative state.
By labeling the tumor cells in genetically engineered mice with inexperienced fluorescent protein (GFP), Joyce and her colleagues noticed how tumors regress and recur. In some circumstances, even a single GFP-positive tumor cell could possibly be seen mendacity in wait within the scar tissue for a lot of months earlier than rising, reactivating proliferation applications and inflicting tumors to rear their ugly heads as soon as extra. “It is in all probability one of many extra hanging findings from the paper, the place you see these photographs and [the cancer cells] are literally within the strategy of leaving the scar,” Joyce added.
These outcomes begged the query: Might scar formation be stopped? And in that case, might recurrence be prevented? Because the group quickly found, this was the difficult half. First, they tried to inhibit remodeling progress issue beta (TGF-β) signaling, which had stood out to them as a key pathway driving fibrosis, in mice. There was no impact.

Professor Johanna Joyce, at present of the College of Lausanne, found why glioblastoma tumors recur so steadily and developed a mixture remedy method to forestall it.
Johanna Joyce, College of Lausanne
Subsequent, they tried a broad-spectrum anti-inflammatory remedy to forestall neuroinflammation pathways, which had equally been highlighted as a driver of fibrosis and scar formation. Once more, they noticed no impact. Even these two therapies together confirmed no discount in scar formation or enhanced survival of the animals.
The breakthrough that they hoped for got here after they tried inhibition of TGF- β signaling and anti inflammatory remedy with the unique macrophage-targeted remedy. “What we discovered is that these three medication together primarily blocked the scars from forming,” Joyce stated. In a long-term preclinical research, all however one mouse that acquired the remedy survived.
An intriguing facet of GBM is that different remedies, together with surgical procedure, chemotherapy, and radiation, additionally generally end in fibrotic scarring and tumor recurrence. One of many subsequent steps for Joyce and her group is to discover the scars that come up from these different remedies to find out if the mechanisms of scar formation and tumor recurrence are the identical and in the event that they, too, could be prevented. The analysis “…might enhance survival charges, that are at present very poor for glioblastoma sufferers,” Jenkins summarized.
References
- Quail DF, et al. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science. 2016;352(6288):aad3018.
- Watson SS, et al. Fibrotic response to anti-CSF-1R remedy potentiates glioblastoma recurrence. Most cancers Cell. 2024;42(9):1507-1527.e11.